Best practices for a successful bioprocess technology transfer
14 June 2021 | Monday | Analysis
Source : Cytivalifescience
What is tech transfer, and when does it happen?
Technology transfer is a formalized process for moving the manufacture of a drug from one facility, one scale or one drug life cycle phase to another.
Tech transfer occurs multiple times during the life cycle of a drug as it progresses from lab to commercial production. At each stage, changes in equipment, methods, and materials may require process optimization or redevelopment, and the new production team will need training. Changes in materials or scale can result in process variability, and critical process parameters may need to be optimized, or the entire process may need to be redeveloped.
Whether you are a well-established biopharma company or a small start-up, smooth tech transfer is critical to the success and speed to market of your drug. A successful tech transfer requires diligent planning, proper documentation and procedures, and experienced experts who can guide the process and avoid the pitfalls.
For a large biopharma company, tech transfer may also occur when they want to add capacity by bringing an additional facility on-line, or wish to transfer production to another site, or even to another country.
Small companies that are developing a new drug and do not yet have manufacturing capabilities may wish to work with a contract manufacturing organization (CMO) or contract development manufacturing organization (CDMO). Established companies with many drugs in the pipeline may also turn to CMOs for manufacturing capacity in the pre-clinical and clinical phases. If the drug is approved and enters the market, companies may then wish to transfer manufacture back in-house or to new facilities.
All of these situations involve technology transfer. There are always risks involved: careful planning and documentation, understanding of critical quality attributes, and with attention to detail at every step of the process, are of paramount importance.
Elements of technology transfer
Let’s look at a stripped-down model of technology transfer and see what it entails.
Knowledge transfer is the heart of technology transfer. Who will be on the receiving team? Do they have the necessary expertise? What training will be required to complete the tech transfer successfully?
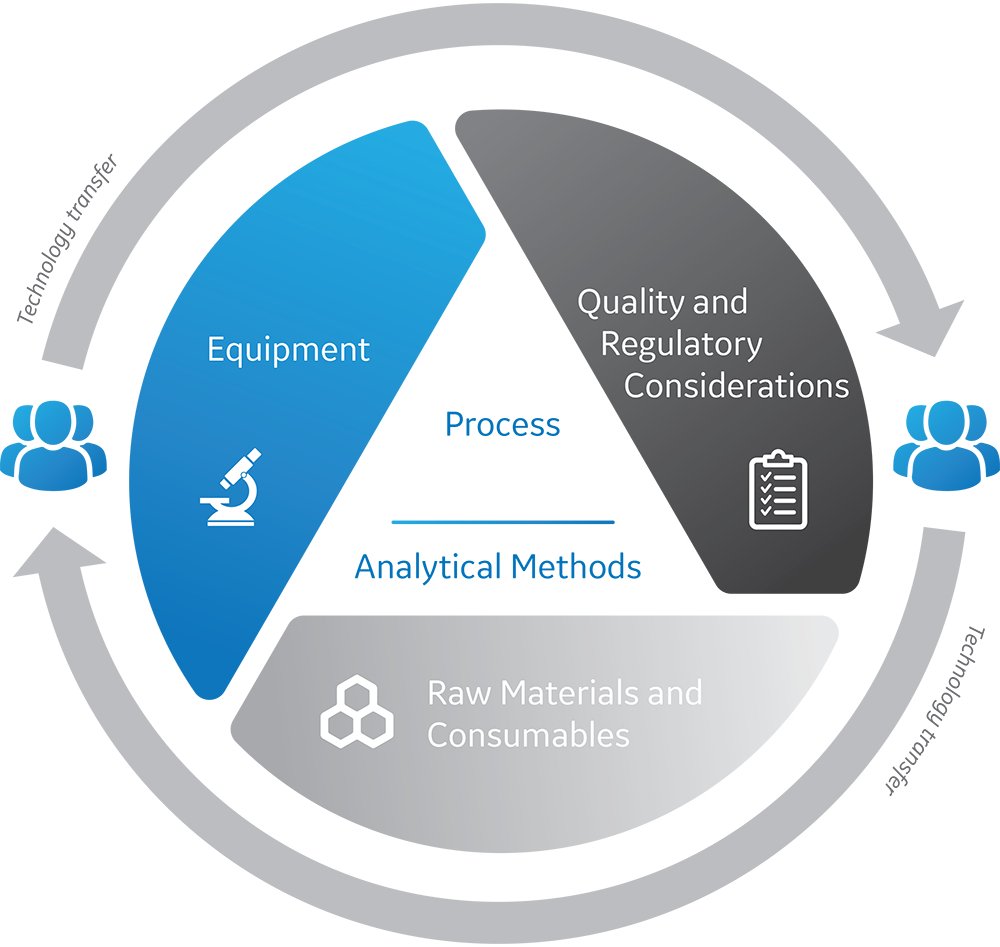
Equipment: It is unlikely that the target facility will have exactly the same equipment and models as the transferring facility. How will these differences affect the process? Will process changes be necessary ?
Raw materials and consumables: If your target facility uses different equipment, are your raw materials and consumables compatible? If you are moving the process to another country, will a secure supply of your media, buffers and resins be available? Are the materials that you are currently using compliant with local regulatory requirements in the new location? If you must substitute, what and how much process re-development will be necessary?
Regulatory considerations: You may need to re-validate your process you are running on different equipment. If you are transferring to a different country, you may need to re-validate not only your process, but also your analytical methods to comply with local regulations.
▻TIP: Remember that tech transfer must also encompass your analytical methods, including characterization testing, release testing and comparability.
But it’s not that simple…
A start-up biopharma company at the late research stage or clinical stage will face additional complicating factors.
- Scaling up from lab-scale to clinical production will entail development work to translate the process. How deeply do you understand your process and mechanism of action (MoA)? What can be transferred directly, what can benefit from a scale transfer function, and what needs to be redeveloped de-novo? The more that you know and share, the smoother the tech transfer is likely to be.
- A lean start-up organization is often lacking in-house expertise in tech transfer and bioprocess manufacturing. You will need expertise “on your side” to ensure that all your bases are covered, and you have oversight from bioprocess quality, business and legal perspectives.
- If outsourcing, CMO selection and relationship will be critical to the success of your tech transfer, your therapy and your business.
▻TIP: Ensure that key expertise in the critical process and manufacturing attributes is retained in your organization to help guide tech transfer.
The top five essential factors for efficient tech transfer
Technology transfer is a significant step along the way to your end goals for your drug or therapy. Make sure these essential factors are in place and aligned with your goals to maximize tech transfer efficiency and success.
- A comprehensive tech transfer document reflecting deep product understanding and includes all the important parameters of your bioprocess, analytical procedures and critical quality attributes (MoA, CQAs etc.)
- CMO capabilities aligned with your requirements and goals
- Clear goals setting up front
- Timelines, costs and deliverables meet your needs
- Expertise and quality oversight in the specific applications required
- Capacity
- Communication – open and often
- Transparency
- Contract SOP embodying a robust plan
- Clearly defined roles and responsibilities
- Process and facility map
- Analytics transfer
- Process development as necessary
- Demonstrated equivalence at the starting scale/process
- Proof of concept engineering runs at scale prior to GMP
- Clear, frequent and transparent communication.
- CMO transparency—being able to go into the plant to see how the process is run, and being able to bring the process back in-house, i.e. (including reverse knowledge transfer).
Document, document, document
Documentation is not an event, but a best practice to be implemented, and referred to, at every step along the way. Templated standard operating procedures and documents, approved by the quality team, will increase the speed, efficiency, effectiveness and quality of technical transfer. Rigorous documentation of deviations along with root cause analysis and corrective/preventative actions will help ensure consistent process execution.
The technology transfer document
From the technology and bioprocess standpoint, the tech transfer document is the all-important roadmap to implementing your process in the CMO’s environment. It will contain all the information and parameters pertaining to the process and the product, including materials and analytical methods. The more that you have nailed down regarding your process, and your effectiveness in documenting this knowledge, the smoother will be your technology transfer and any ensuing redevelopment.
The technology transfer SOP
Since technology transfer is core to the business of a CMO, you can expect your CMO or CDMO to work within the framework of a clearly-defined methodology, documented as a standard operating procedure (SOP). The goal of the technology transfer SOP is to improve the effectiveness, efficiency, quality and compliance of the biomanufacturing technology transfer process. A thorough, reliable tech transfer methodology will go a long way towards ensuring success in both the technical aspects and in building the business relationship.
Expect a technology transfer documented SOP based off a standard methodology and templates, tailored to the specific project. A well-designed SOP will include:
- Defining the goals, scope and steps involved
- Defining roles and responsibilities
- Utilization of template documents
- Increasing the level of control through process control, document control, communication, decision and sign-off controls
- Increasing the level of quality through quality team involvement and oversight
If your tech transfer is to a CMO, the contract with the CMO will be driven by SOP and the technology transfer document, so be sure the SOP is detailed and robust.
CMO or CDMO selection: key considerations
Selecting your CMO or CDMO, and crafting your contract with them, is one of the most critical and complex business decisions for your company. You will be sharing your intellectual property with them, and they, in turn, will be creating significant intellectual property as they scale up your drug and continue its development. Be sure to include reputation, as well as capabilities, in your selection criteria. How can you prepare in advance to avoid typical pitfalls?
▻TIP: Hire an experienced expert who can guide you through vendor selection and contracting, who knows what to look for, and what to look out for. It is well worth the investment.
Here are some of the key factors that go into framing your vendor selection and contract.
- Timelines: do they have capacity to meet your timelines? Can you secure additional runs if demand exceeds forecast?
- Equipment: how similar, or different, is their equipment to what you are currently using? Is their equipment in line with your long-term plans? For example, if you foresee eventual commercial production with single-use equipment, is their current equipment aligned?
- Expertise: Do they have experience working with similar drugs?
- What do they bring to the table for analytical procedures? If you later transfer production, will you have access to their methods and SOPs for both analytical and manufacturing?
- Regulatory considerations: what do they bring to the table, and what do you need? Can they meet clinical regulatory requirements, and can they go beyond that to provide guidance for you?
Understanding where you are, and where you are going
Planning a successful tech transfer requires understanding what both sides (sending and receiving) have for process and equipment, the differences, and what redevelopment might be required.
▻TIP: Consider the definition of your end-point carefully. While you may be moving from lab to scale-up for pre-clinical production or Phase I trials, is that really your end-point? Choices (e.g. equipment, consumables) at any stage may simplify or add complexity to future tech transfers, scale up and redevelopment activities.
Intellectual property considerations
For a small bio-pharma start-up, especially one spun out of a research lab, hiring a consultant with experience in negotiating successful biomanufacturing outsourcing contracts will be a wise investment. Framing all of the aspects of the business relationship, the contract will help set expectations and avoid future pitfalls.
Don’t overlook the intellectual property that will be developed or supplied by the CMO. This may include analytical methods, cell lines that must be licensed, documentation of their process and methods. Should you wish to bring manufacturing in-house, or transfer to a different or additional CMO in the future, will this IP be available to you? What costs will be associated with it?
▻TIP: Remember that when working with a CMO or a CDMO, not only will you be sharing your IP with them, but they, also, may integrate their own IP into the manufacture (and testing) of your drug, as well as potentially develop new IP and you should plan for this eventuality.
Engineering runs
The engineering run is a non-GMP trial run which tests your process, equipment and automation to confirm that your process is outlined correctly , as well as confirming that the staff is properly trained to run the process. During an engineering run, your process will be run at scale, or close to at scale, on the CMO equipment prior to transitioning to GMP production. While this can be quite costly, it is worth the expense. During this run, any differences in the process resulting from scale, materials or equipment differences will be exposed. Identified critical process parameters may need to be adjusted. The learnings gained during an engineering run will help drive a lot of risk out of the transition to a GMP manufacturing process. If you go straight into GMP manufacturing and run into any issues, trouble-shooting can be much costlier—in time and money—than the engineering runs.
Transparency
Technology transfer is a complex undertaking that requires communication, and even collaboration, between the biopharma company and the CMO . Transparency is a key factor, so understand your CMO’s style up front to set clear expectations. For example, what is their policy and preference regarding a “person-on-the-floor” from the client side? Will you be able to go into plant to see how the process is run? This is especially important if you plan to bring the process back in-house.
▻TIP: A relationship is a two-way street. Build trust with clear expectations, communications and transparency.
Best practices: wrap it up
It may be tempting to think of technology transfer as a “send and receive” process, with the sending organization transferring technology to the receiving organization. It will be more helpful, and set you up for success, to understand tech transfer in the framework of a relationship, whether between the biopharma company and their CMO, or between different teams internally.
When working with a CMO, the tech transfer, while a major milestone, is not just a hand-off—it is the beginning of an ongoing relationship. It is important to establish a strong, cooperative rapport with the CMO’s alliance manager, and to maintain quality oversight on an ongoing basis. Throughout the tech transfer process, you will be defining, establishing and building this relationship. For a start-up or emerging pharma company, this is a bet-your-business prospect.
- Utilize consultant(s) to guide you through.
- Plan for contingencies, plan for success. Early planning prevents later grief.
- Cover all the bases, including supply chain, equipment, regulatory and intellectual property.
- Manage the vendor selection criteria and process with expert help.
- Document tech transfer in depth:
- Technology transfer document from the SENDING side
- Technology transfer standard operation procedure (SOP) from the RECEIVING side, adapted to the specific instance
- Contract, from both sides
As in the bioprocess lab, in tech transfer the devil is in the details. Nail down as much as possible up front, but communications and transparency are necessary to deal effectively with the many issues that may arise along the way.
Most Read
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Avantor’s New CEO Ligner Aims to Unlock Global Potential and Deliver Shareholder Value
- AstraZeneca Commits $50 Billion to U.S. Expansion by 2030 in Biggest-Ever Global Investment
- Thermo Fisher, SAMRC, and South Africa’s Department of Science and Innovation Launch CATIR to Nurture Next-Gen Scientists
- Cube Biotech Appoints Former Sartorius CEO Dr. Joachim Kreuzburg to Board of Directors
- FDA’s AI Transition Marks a Turning Point in Drug Review: Industry Faces Pressure to Adapt Amid 20% Workforce Cut
- WuXi XDC Completes Mechanical Build of Singapore Bioconjugate Manufacturing Hub
News
Editor Picks











