LifeScience BioSimilar
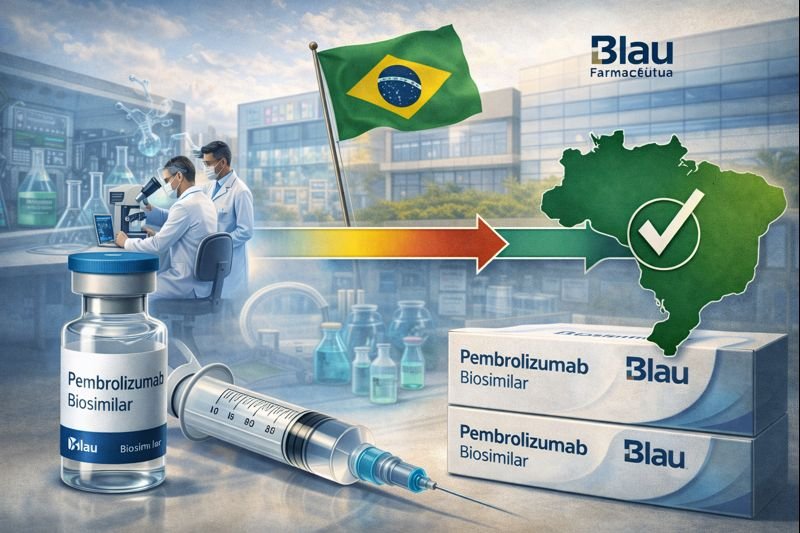
Blau Farmacêutica Achieves End To End Development Of Pembrolizumab Biosimilar In Brazil
Blau Farmacêutica, a Brazilian pharmaceutical company listed on Brazilian Stock Market (B3) and a regional leader in hospital pharmaceuticals w...
January 27, 2026 | News
Samsung Bioepis Takes Full Control Of BYOOVIZ Commercialisation In Europe
Samsung Bioepis completes full transfer of commercial rights from Biogen back to Samsung Bioepis for BYOOVIZ® BYOOVIZ becomes the fourth biosimila...
January 05, 2026 | News
EMA CHMP Backs Gotenfia Golimumab Biosimilar Advancing STADA and Bio Thera European Strategy
Positive opinion from European Medicines Agency supports approval for golimumab biosimilar Gotenfia® developed by Bio-Thera and to be marke...
December 30, 2025 | News
Bioeq And Zydus Secure USFDA Approval For Interchangeable Lucentis Biosimilar NUFYMCO
Bioeq AG ("Bioeq"), a Swiss biopharmaceutical company, and Zydus Lifesciences Limited ("Zydus"), an innovation-led life-sciences company with an internatio...
December 24, 2025 | News
Samsung Bioepis Gains CHMP Backing for BYOOVIZ Pre Filled Syringe
European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) adopts positive opinion for BYOOVIZ® (ranibizuma...
December 03, 2025 | News
Celltrion Gains EU Approval for Omlyclo 300 mg Prefilled Syringe as First Omalizumab Biosimilar
Omlyclo™ (omalizumab) is the first and only omalizumab biosimilar approved in Europe Omlyclo™ 300 mg/2ml prefilled syringe (PFS) presentat...
November 19, 2025 | News
Shanghai Henlius and Organon Achieve FDA Approval for POHERDY, First Pertuzumab Biosimilar in the US
Shanghai Henlius Biotech, Inc. (2696.HK), and Organon (NYSE: OGN) announced that the US Food and Drug Administration (FDA) has approved the Biologics...
November 19, 2025 | News
EirGenix and Sandoz Sign USD 152 Million Global Deal for Pertuzumab Biosimilar
EirGenix Inc. announced that it has entered into a second global exclusive licensing agreement with international biosimilar leader Sandoz AG (SIX:SDZ/OTCQ...
November 13, 2025 | News
Samsung Bioepis Reaches Settlement with J&J on Stelara Biosimilar PYZCHIVA in Europe
Samsung Bioepis Co., Ltd. has signed a settlement and license agreement with Johnson & Johnson concerning the commercialization of PYZCHIVA®, a bio...
November 07, 2025 | News
Samsung Epis Holdings Established as New Biotechnology Investment Holding Company Following Spin-Off from Samsung Biologics
Samsung Epis Holdings Co., Ltd.announced its establishment as a new investment holding company, following the spin-off of Samsung Bioepis Co., Ltd. from Sa...
November 04, 2025 | News
Celltrion Receives U.S. FDA Interchangeability Designation for Denosumab Biosimilars STOBOCLO® and OSENVELT®
The U.S. FDA approved STOBOCLO® (denosumab-bmwo) and OSENVELT® (denosumab-bmwo) as interchangeable with the ref...
November 03, 2025 | News
Celltrion Receives FDA Approval for EYDENZELT®, a Biosimilar to EYLEA® for Retinal Diseases
EYDENZELT® is approved for the treatment of patients with neovascular (wet) age-related macular degeneration (wAMD), macular edema following ret...
October 13, 2025 | News
FDA Approves Henlius and Organon’s Denosumab Biosimilars BILDYOS and BILPREVDA for All US Indications
Shanghai Henlius Biotech, Inc. and Organon announced that the US Food and Drug Administration (FDA) has approved BILDYOS® (denosumab-nxxp) inject...
September 04, 2025 | News
Abbott Secures Thailand Approval For Denosumab Biosimilar Expanding Access To Osteoporosis Treatment
Abbott denosumab biosimilar has received regulatory approval in Thailand, expanding access to advanced therapies for osteoporosis and cancer-related bone l...
August 27, 2025 | News
Most Read
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Avantor’s New CEO Ligner Aims to Unlock Global Potential and Deliver Shareholder Value
- AstraZeneca Commits $50 Billion to U.S. Expansion by 2030 in Biggest-Ever Global Investment
- Thermo Fisher, SAMRC, and South Africa’s Department of Science and Innovation Launch CATIR to Nurture Next-Gen Scientists
- Cube Biotech Appoints Former Sartorius CEO Dr. Joachim Kreuzburg to Board of Directors
- FDA’s AI Transition Marks a Turning Point in Drug Review: Industry Faces Pressure to Adapt Amid 20% Workforce Cut
- WuXi XDC Completes Mechanical Build of Singapore Bioconjugate Manufacturing Hub
News
Editor Picks