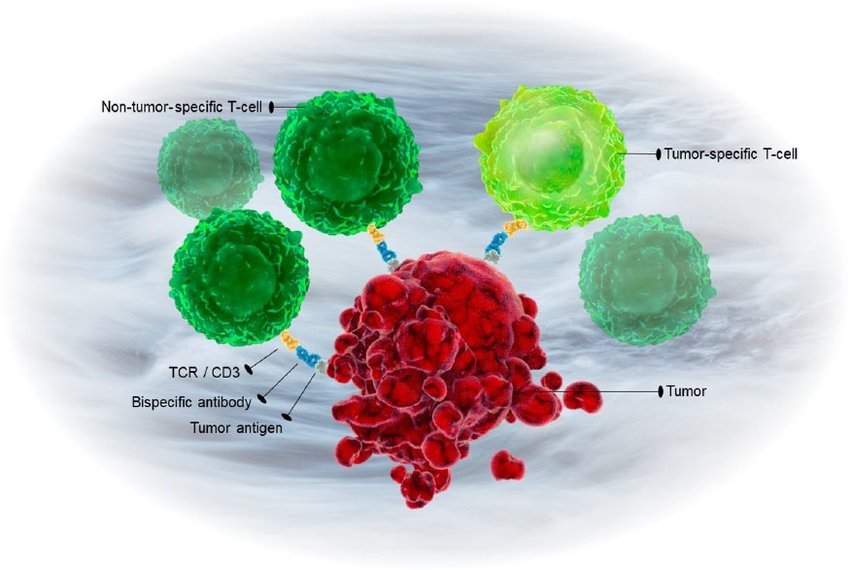
BioPharma Drug Approval
Bridge Biotherapeutics Receives FDA Approval for First-in-Human Study of BBT-207, a 4th Gen EGFR TKI.
The company has been notified by the FDA that the Phase 1/2 clinical trial may proceed The latest preclinical data explored the antitumor effica...
April 24, 2023 | News
Quizartinib NDA Review for Patients with Newly Diagnosed FLT3-ITD Positive AML Extended by FDA
The FDA has extended the Prescription Drug User Fee Act (PDUFA) action date by three months to July 24, 2023 to allow additional time to review requested u...
April 21, 2023 | News
CARsgen's CT041 receives IND clearance from NMPA for pancreatic cancer therapy.
Dr Raffaele Baffa, Chief Medical Officer of CARsgen, commented that "We are glad to receive the IND clearance from NMPA for the adjuvant treatment of ...
April 20, 2023 | News
Ajinomoto Bio-Pharma Services Receives FDA Approval for High Potency Fill Line
"Receiving FDA approval on our HPAPI fill line is an exciting milestone for our company, and couldn't have happened without the hard work, hours of prepara...
April 20, 2023 | News
Crystal Formulation Services Receives China Drug Manufacturing License for Milestone Formulation Capability
Crystal Formulation Services, a subsidiary of Crystal Pharmatech, has achieved an exciting new milestone in its formulation capabilities. The company was r...
April 20, 2023 | News
Biosyngen announces FDA IND approval of its second product for EBV-positive lymphoma
A few months earlier, the Company's first product (BRG01) targeting relapsed/metastatic nasopharyngeal cancer has been granted IND approval by both the US ...
April 19, 2023 | News
FDA Accepts Supplemental Biologics License Application (sBLA) for KEYTRUDA® (pembrolizumab)
Merck (NYSE:MRK), known as MSD outside the United States and Canada, today announced the U.S. Food and Drug Administration (FDA) has accepted for...
April 17, 2023 | News
Minghui Pharma's MH004 Cream Succeeds in Phase 2 Trial for Atopic Dermatitis, Global Phase 3 MRCT Cleared by FDA
Study met both primary and all key secondary endpoints Mean percentage change from baseline in Eczema Area and Severity Index (EASI) score was -78.7% in...
April 14, 2023 | News
Biosyngen received China NMPA IND approval for its T-cell redirection therapy targeting EBV-positive Lymphoma
Currently, commercially available cell therapies, such as CD19 CAR-T, are designed for the treatment of B cell lymphoma or acute lymphoblastic leukemia. Tr...
April 12, 2023 | News
Boan Biotech's Biologics License Application for Boyounuo® (Bevacizumab Injection) Accepted in Brazil
Envisioned to be "a leading global biopharmaceutical company", Boan Biotech develops biologics for both Chinese and international markets, including E...
April 12, 2023 | News
JW Therapeutics' Relma-cel approved for systemic lupus erythematosus trial.
SLE is a complex autoimmune disease with diverse clinical manifestations involving many organs and systems. It is estimated that China has 1 mill...
April 11, 2023 | News
Chinese NMPA Approves Study of Telix Brain Cancer Therapy Candidate
The investigational new drug (IND) application was submitted by Telix's partner in the Greater China region, Grand Pharmaceutical Group Limited (...
April 11, 2023 | News
Tillotts Pharma AG announces the launch of Octasa® 1600 mg tablets in Canada
The expansion of the Octasa® family in Canada with the launch of Octasa® 1600 mg represents an important step in the strategic partnership of Tillo...
April 07, 2023 | News
Leica Biosystems Strengthens Portfolio with FDA Clearance of Class II Mismatch Repair (MMR) Panel
Leica Biosystems announced the US Food and Drug Administration (FDA) 510(k) clearance of the BOND MMR Antibody Panel, providing customers with a high-perfo...
April 04, 2023 | News
Most Read
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News