Medical Devices
FDA Designates Amadix's Blood Test as Breakthrough Device for Colorectal Cancer Screening
PreveCol®, a blood test for oncological diagnosis, has obtained US recognition for its efficacy in detecting precancerous lesions compared to existin...
January 24, 2024 | Regulatory
Fujirebio and Agappe Collaborate on CLIA-Based Immunoassay
In a significant move, Agappe and Fujirebio have revealed plans to launch these products in June 2024. The project has already achieved significant progres...
January 22, 2024 | News
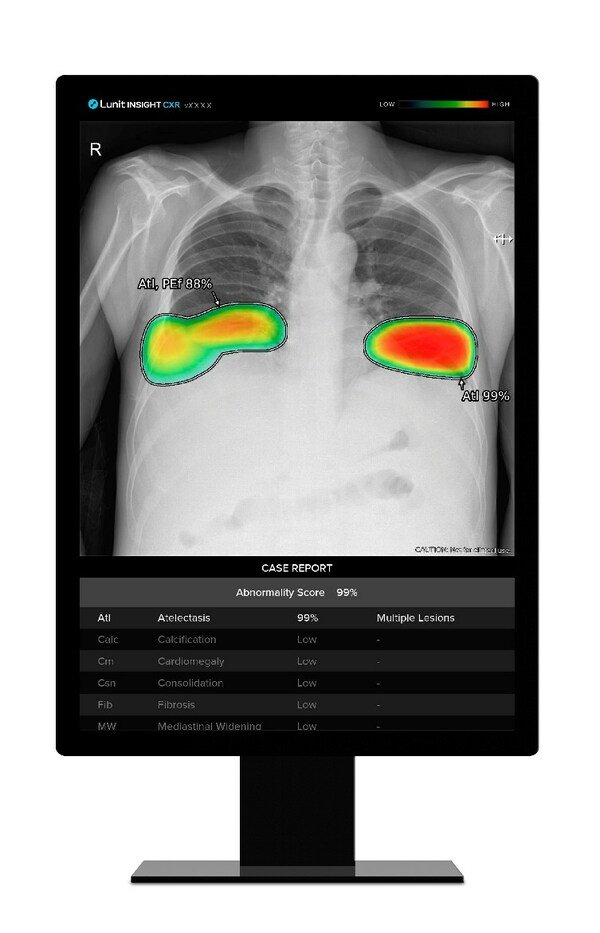
Lunit AI to Enhance Samsung's X-ray Devices for Chest Screening
Three-year supply contract: Lunit INSIGHT CXR and Lunit INSIGHT CXR Triage to enhance lung abnormality detection in Samsung's premium X-ray devices &nbs...
January 17, 2024 | News
CardioFocus Acquires Galvanize Therapeutics' Electrophysiology Division
Acquisition includes CE- Marked CENTAURI™ Pulsed Field system Accelerates CardioFocus' development of the HeartLight X4 true single s...
January 12, 2024 | News
Alcresta Therapeutics Receives FDA Clearance for RELiZORB® Cartridge
Alcresta Therapeutics, Inc., a leading commercial-stage company focused on developing and commercializing novel enzyme-based products, announced 510(...
January 02, 2024 | News
Exactech Announces FDA 510(k) Clearance of the World’s First Surgical Navigation for Total Ankle Replacement
GPS Ankle is a first-of-its-kind technology, which connects the preoperative plan with real-time intraoperative instrument guidance and confirms that resec...
December 06, 2023 | News
Lunit Advances Singapore's Healthcare with AI-Powered Medical Imaging Solution.
Lunit (KRX:328130.KQ), a leading provider of AI-powered solutions for cancer diagnostics and therapeutics, partners with NTT DATA Singapore to introduce Lu...
December 01, 2023 | News
Lunit INSIGHT DBT receives FDA 510(k) clearance, poised to enter the world's largest DBT market
Lunit a leading provider of AI-powered solutions for cancer diagnostics and therapeutics, announced that it has received U.S. Food and Dr...
November 15, 2023 | News
908 Devices and Terumo Collaborate on Quantum Flex Cell Expansion System Analytics
908 Devices Inc. (Nasdaq: MASS), a pioneer of purpose-built handheld and desktop devices for chemical and biochemical analysis, and medical technology...
November 07, 2023 | News
Huawei's Finnish Health Lab Advances Global Health & Fitness Research
Sized at almost 1000 square metres, the laboratory is equipped with a diverse range of world class sports equipment covering over 20 types of sports. Each ...
October 31, 2023 | News
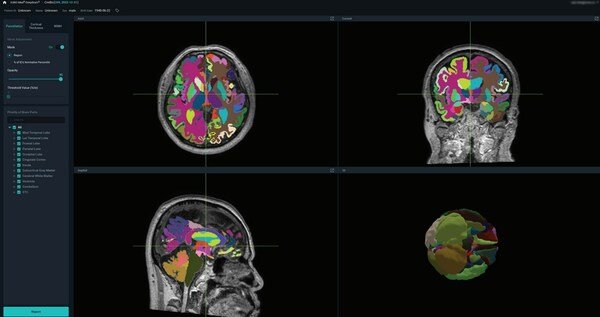
VUNO Secures FDA 510(k) Clearance for VUNO Med®-DeepBrain®
VUNO Med®-DeepBrain® is intended to automate the current manual process of identifying, labeling, and quantifying segmentable brain structures from...
October 24, 2023 | News
Smith+Nephew Unveils Innovative Surgical Training Center in Munich
S+N Academy Munich, situated in Kustermann Park in the heart of the city, will provide a central European hub for surgeons from across Europe, the&nbs...
October 12, 2023 | News
GE HealthCare Expands Human Subject Research with Stanford Medicine Using Photon Counting CTi Technology
Unique from current CT technology, GE HealthCare’s proprietary photon counting CT design is engineered to leverage Deep Silicon detectors with the ...
October 10, 2023 | News
Nortech Systems Suzhou Facility Certified to Produce Class II Medical Devices for Asia Market
“NMPA certification is a major step forward for Nortech Systems and our customers in the region,” said Nortech Systems CEO, Jay D. Miller. &ldq...
October 05, 2023 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News