Jiangsu Vcare and Huadong Medicine Form Exclusive Partnership for Commercialization of VC005 Tablets in China
18 August 2025 | Monday | News
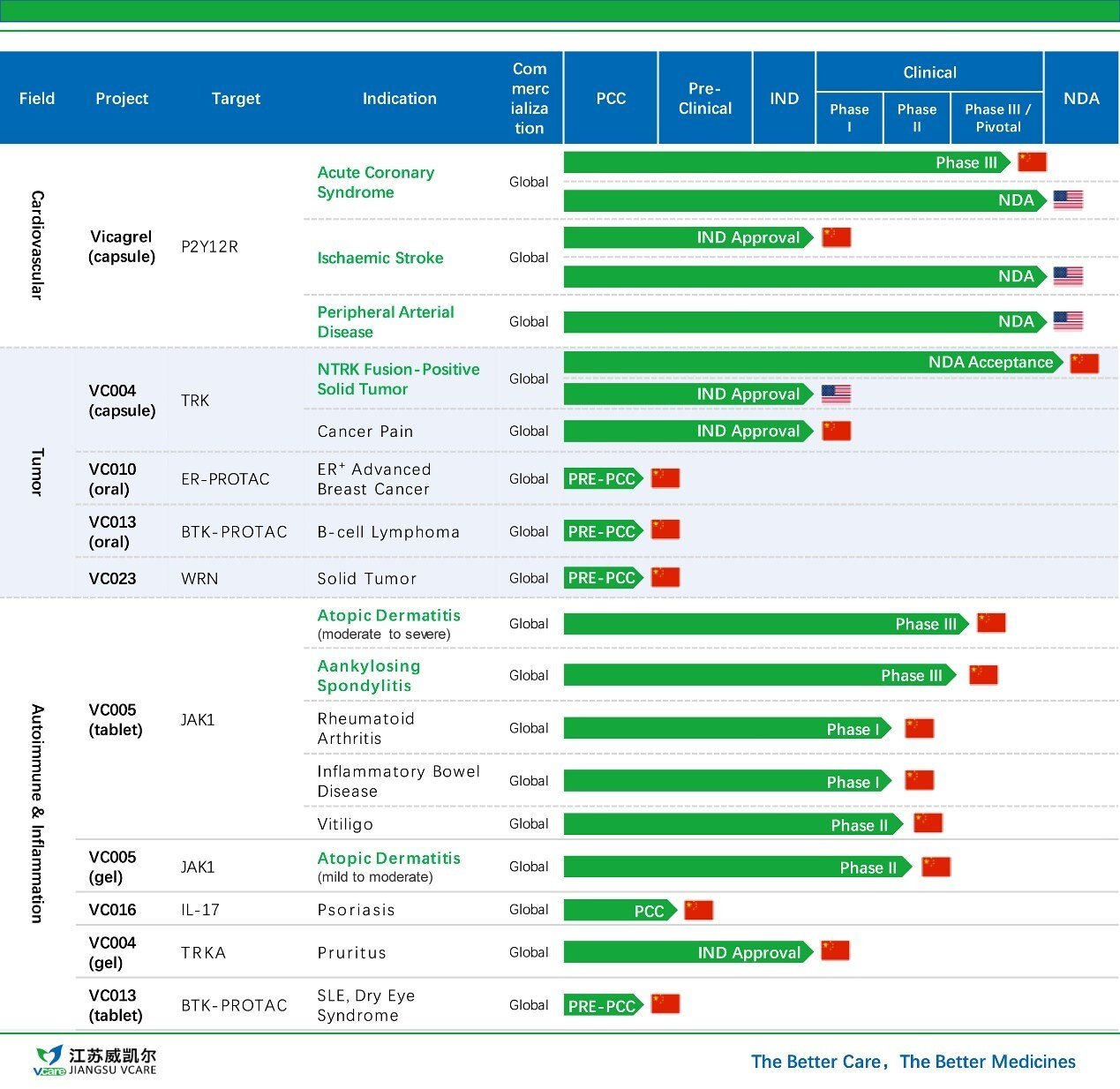
Jiangsu Vcare’s Pipeline
Jiangsu Vcare Pharmatech Co., Ltd. (Jiangsu Vcare) announced that it has entered into an exclusive strategic partnership with Huadong Medicine (Hangzhou) Co., Ltd., a wholly-owned subsidiary of Huadong Medicine Co., Ltd. (000963.SZ, "Huadong Medicine"), for the commercialization rights of Jiangsu Vcare's innovative product VC005 tablets in mainland China.
Under the agreement, Jiangsu Vcare will remain the marketing authorization holder (MAH) and retain responsibility for R&D, registration, manufacturing, and supply of VC005 tablets. Jiangsu Vcare will receive an upfront payment of RMB 50 million and registration milestone payments of up to RMB 180 million. Huadong Medicine will take charge of commercialization and market promotion across mainland China.
VC005 tablet is a second-generation, highly selective JAK1 inhibitor independently developed by Jiangsu Vcare. Designed to treat multiple autoimmune diseases, the therapy is currently is being evaluated in a phase III trial for moderate-to-severe atopic dermatitis in China, with phase III for ankylosing spondylitis and Phase II trial for vitiligo are being initiated.
Dr. Gong Yanchun, Co-founder and General Manager of Jiangsu Vcare, stated: "As one of Jiangsu Vcare's core clinical-stage assets, VC005 tablets represent a significant strategic move in the autoimmune filed. This collaboration with Huadong Medicine underscores the strong commercial potential of our self-developed innovative drugs, while accelerating their pathway to market and maximizing both clinical and commercial value. By combining VC005's differentiated clinical advantages with Huadong Medicine's robust commercialization expertise, we are confident this win-win partnership will bring the therapy to more patients and achieve broad market recognition."
Mr. Lv Liang, Chairman and General Manager of Huadong Medicine, added: "The autoimmune field is a core strategic priority for Huadong Medicine. VC005 tablets, with their potential to address multiple autoimmune conditions including atopic dermatitis, ankylosing spondylitis, and vitiligo, will further strengthen our leading position in China's autoimmune market. Leveraging our nationwide market coverage and deep experience in this therapeutic area, we believe VC005 will achieve strong market penetration and deliver meaningful benefits to patients."
Most Read
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











