WhitePaper
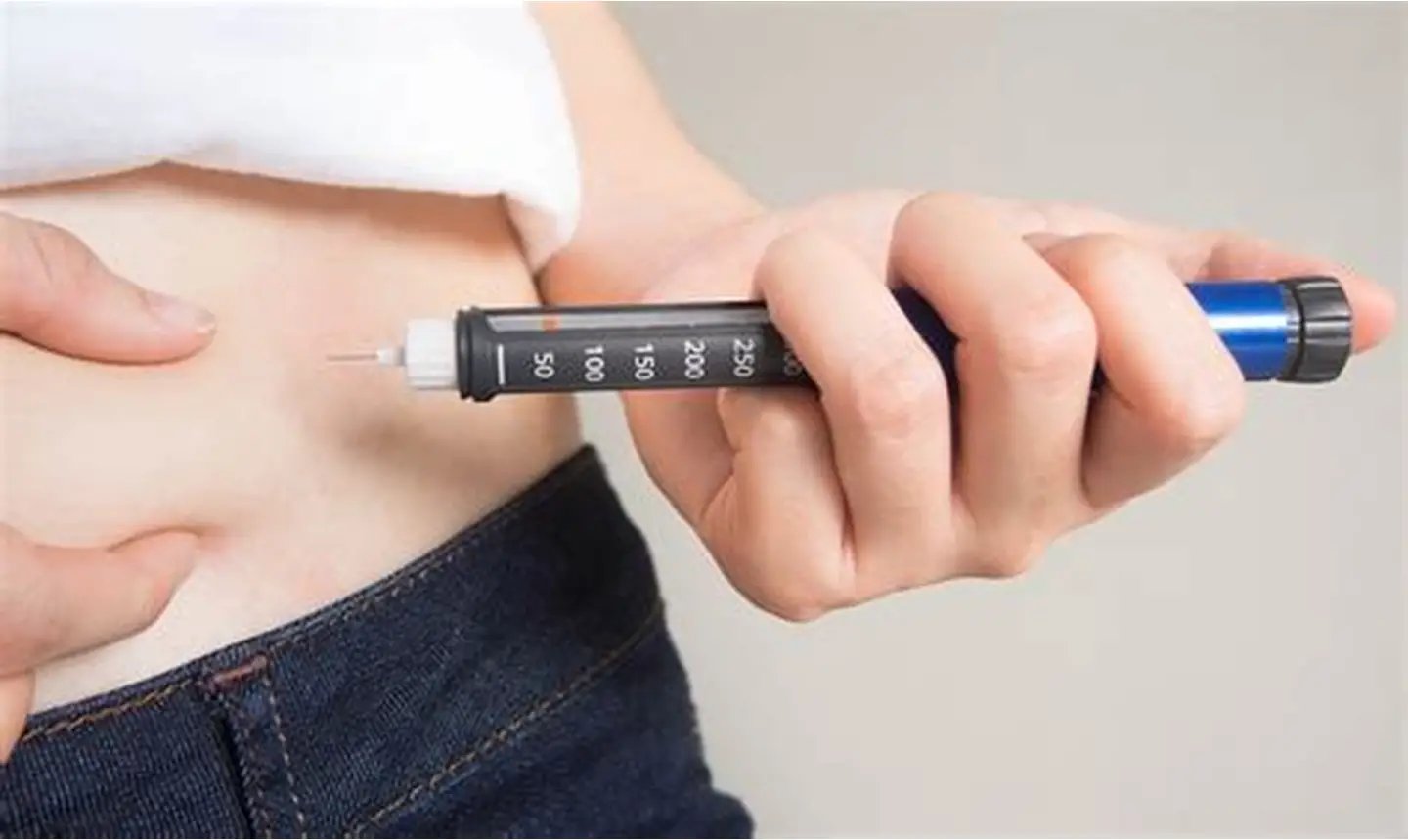
How Does GLP-1 Work?
From trendy diets to relentless gym schedules, the quest for shedding those stubborn pounds is a challenge many people face at some point in their lives. I...
February 17, 2026 | News
New England Biolabs Showcases Automation Ready NGS Workflows Through Global SLAS Collaborations
New England Biolabs (NEB ®) will present several scientific posters at this week's Society for Laboratory Automation and Screening (SLAS) meeting in Bo...
February 09, 2026 | News
26 Key Trends Shaping Biotech And Life Sciences In 2026
As 2026 unfolds, biotech and life sciences are moving into a phase defined less by promise and more by execution. Faster R and D cycles, smarter cl...
January 05, 2026 | News
2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
At the centre of this momentum stood the U.S. Food and Drug Administration, whose 2025 approvals illustrated how medicine is moving decisiv...
December 29, 2025 | Analysis | By arcilla.fran@biopharmaapac.com
BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
The year 2025 has marked a pivotal moment for the biopharmaceutical industry as it reckons with the aftershocks of a global pandemic and ongoing geopolitic...
December 24, 2025 | Analysis
APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
APAC Biopharma in Review: What Truly Moved the Needle This Year Asia-Pacific (APAC) has cemented its status as a pivotal biopharmaceutical hub in 2025, ma...
December 15, 2025 | Report
Top 25 Biotech Innovations Redefining Health And Planet In 2025
Biotechnology in 2025 is no longer confined to the laboratory bench. It is curing inherited blood disorders, changing how we prevent HIV, reimagining Alzhe...
December 10, 2025 | Report | By BioPharma APAC Wrap Up 2025
The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
Global pharmaceutical companies are racing to tap China’s new AI-powered biotech platforms to discover drugs faster and cheaper. In 2025, major Weste...
November 27, 2025 | Analysis
Single-Use Systems Are Rewiring Biopharma Manufacturing
Bioprocess, Unbottled Single-use systems (SUS) have become a transformative force in biopharmaceutical processing, offering an alternative to trad...
September 16, 2025 | Report
The State of Biotech and Life Science Jobs in Asia Pacific – 2025
In 2025, Asia-Pacific has emerged as the fastest-growing hub for biotech and life sciences employment, fuelled by a powerful combination of government supp...
September 09, 2025 | Report
Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
The first eight months of 2025 have been transformative for biosupplier technologies worldwide. From innovative lab reagents and biomaterials to ad...
August 22, 2025 | Analysis
Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Invisible chemical impurities in medicines can pose very visible risks to patient safety and pharmaceutical reputations. In recent years, a quiet c...
August 19, 2025 | Analysis
China’s Biopharma Dealmaking Surges in H1 2025, Driven by Record Licensing and Oncology Focus
The first seven months of 2025 saw robust dealmaking in China’s biopharma and healthcare sector, even as global headwinds tempered some activity. Chi...
August 15, 2025 | Report
Bridging Real-World Evidence and AI: Unlocking Smarter, Faster Clinical Research in Asia
Having worked with pharma clients, healthcare providers, and digital platforms across the Asia-Pacific region, I’ve seen the challenges first-hand&md...
August 04, 2025 | Opinion
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News