DiscGenics Announces Positive Two-Year Clinical Data from U.S. Study of Discogenic Progenitor Cell Therapy for Degenerative Disc Disease
25 January 2023 | Wednesday | News
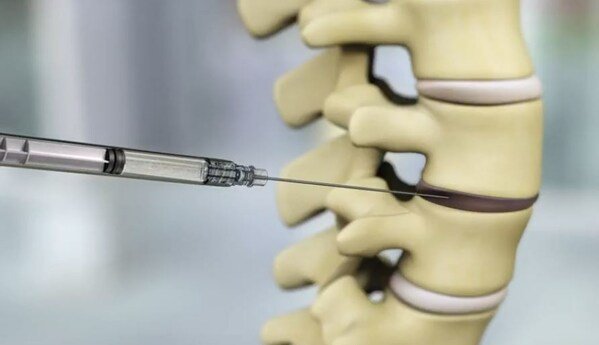
During treatment, a single dose of IDCT is injected into the painful disc percutaneously.
DiscGenics, Inc., a clinical stage biopharmaceutical company focused on developing regenerative cell-based therapies that alleviate pain and restore function in patients with degenerative diseases of the spine, today announced positive two-year clinical data from its first-in-human clinical study of IDCT (rebonuputemcel), an allogeneic discogenic progenitor cell therapy for lumbar degenerative disc disease (DDD).
In the FDA-allowed prospective, randomized, double-blind, vehicle- and placebo-controlled, multicenter clinical study, high dose IDCT (9,000,000 cells/mL; n=20) met the primary safety and efficacy endpoints and demonstrated statistically significant improvements in low back pain, function, quality of life, and disc volume, suggesting a regenerative effect following a single injection into the intervertebral disc.
Key findings include:
- The primary safety endpoint of the study was achieved with no subjects in the IDCT treatment groups experiencing treatment-emergent serious adverse events (TESAEs).
- As previously reported, the primary efficacy endpoint of the study was achieved, with statistically significant improvement in back pain scores by >30% as measured on a 100mm Visual Analog Scale (VAS) observed in the high dose IDCT group at 52 weeks (–62.79%, p=0.0005). A smaller, significant decrease in VAS was also observed in the vehicle group.
- Clinically meaningful, statistically significant improvements in low back pain (VAS), function (ODI), and quality of life (EQ-5D) were observed by 12 weeks following intradiscal injection with high dose IDCT in subjects with symptomatic lumbar disc degeneration.
- These clinical improvements were sustained at six months, one year, 1.5 years, and two years post-injection and statistically exceeded the Minimal Clinically Important Difference (MCID) in each respective outcome measure, which reflect changes following a clinical intervention that are meaningful for the patient.
- In the low dose IDCT group (3,000,000 cell/mL; n=20), there was a trend in improvement of clinical outcomes, though inconsistent. While the vehicle control group (n=10) resulted in some pain relief, it was not associated with clinically meaningful improvements in function or quality of life. No consistent or durable statistically significant or clinically meaningful outcomes were observed in the saline placebo control group (n=10).
- Statistically significant improvements in disc volume were also observed in the high dose IDCT group, where MRI imaging-derived mean change in disc volume increased steadily from baseline and reached statistical significance at Week 52 (249.01 mm3, p=0.0284) and Week 104 (402.1 mm3, p=0.028).
- In contrast, changes in disc volume for the control groups decreased, although not at a statistically significant level.
- Importantly, the high dose IDCT treatment group was the only group in this study to show a decrease in both opioid and nonsteroidal anti-inflammatory drug (NSAID, e.g. aspirin, ibuprofen, etc.) use.
- At 2 years, overall patient follow-up was 85.0%.
"These clinical results demonstrate the incredible potential of DiscGenics's IDCT to safely treat not only the pain and disability associated with DDD with a single injection, but also to address the underlying cause of the disease—the degenerating disc. This is unlike any treatment I have seen in 30 years of practice and unlike any treatment currently available on the market," said Matthew F. Gornet, M.D., Board Certified Spine Surgeon at The Orthopedic Center of St. Louis and top enroller in the IDCT study. "The improvements we observed in disc volume through MRI image analysis suggest DiscGenics's IDCT produces a regenerative effect within the degenerating disc which indicates the ability to halt and possibly reverse the progression of DDD."
The 60-subject study was designed to evaluate the safety and preliminary efficacy of IDCT for the treatment of symptomatic lumbar degenerative disc disease versus vehicle and saline controls. Subjects were enrolled at 13 centers across 12 states.
In this study, low back pain was measured on a 100-mm Visual Analog Scale (VAS), function was measured via the Oswestry Disability Index Questionnaire (ODI), and quality of life was measured using the EQ-5D Index Score.
"We are very encouraged by the final two-year results of this study," said Flagg Flanagan, Chief Executive Officer and Chairman of the Board for DiscGenics. "The significant and durable improvements we saw in pain, function, quality of life, disc volume, and concomitant pain medication usage are critical indicators of the potential for IDCT to change the paradigm of care for patients with DDD."
DiscGenics has submitted a full clinical study report to the U.S. Food & Drug Administration's (FDA) Office of Tissues and Advanced Therapies (OTAT).
Simultaneously, DiscGenics is scaling up its in-house manufacturing capabilities so it will have cells ready for future application, pending the FDA's review of the data.
A summary of this data has been presented at:
- The American Academy of Neurological Surgery (AAcNS) 84th Annual Meeting by Kevin T. Foley, MD, Professor of Neurosurgery at the University of Tennessee Health Science Center and Chairman of Semmes-Murphey Clinic on September 29, 2022.
- The North American Spine Society (NASS) 37th Annual Meeting by Matthew F. Gornet, MD, Board Certified Spine Surgeon at The Orthopedic Center of St. Louis on October 12, 2022.
- The 41st Annual J.P. Morgan Healthcare Conference by Flagg Flanagan, CEO and Chairman of DiscGenics on January 11, 2023.
About IDCT
Most Read
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Top 25 Biotech & Biopharma Leaders in Sustainable Innovation, 2025
- China’s Biopharma Dealmaking Surges in H1 2025, Driven by Record Licensing and Oncology Focus
- Chikungunya in China: How a “Forgotten” Arbovirus Found the Perfect Storm
- How Innovation Gaps in Biopharma Raise New Safety Concerns
- Smart Implants and the Future of Musculoskeletal Injury Treatment
- How Ethical Gaps in Psychiatry Could Undermine Biopharma Progress
- The Evolving Landscape of Women’s Health Innovation in the Asia-Pacific
- Using NLP-Driven Decision Support in Emergency Health Assistance
- Taiwan Steps Into the Global Spotlight With a New Cancer Therapy
- The Role of Unique Device Identification (UDI) in Tracing Medical Device Safety
- The Importance of a Patient’s Mental Health During Clinical Trials
Bio Jobs
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Avantor’s New CEO Ligner Aims to Unlock Global Potential and Deliver Shareholder Value
- AstraZeneca Commits $50 Billion to U.S. Expansion by 2030 in Biggest-Ever Global Investment
- Thermo Fisher, SAMRC, and South Africa’s Department of Science and Innovation Launch CATIR to Nurture Next-Gen Scientists
- Cube Biotech Appoints Former Sartorius CEO Dr. Joachim Kreuzburg to Board of Directors
- FDA’s AI Transition Marks a Turning Point in Drug Review: Industry Faces Pressure to Adapt Amid 20% Workforce Cut
- WuXi XDC Completes Mechanical Build of Singapore Bioconjugate Manufacturing Hub
News
Editor Picks











