Multiply Labs Joins forces with Cytiva to automate cell therapy manufacturing.
26 May 2021 | Wednesday | News
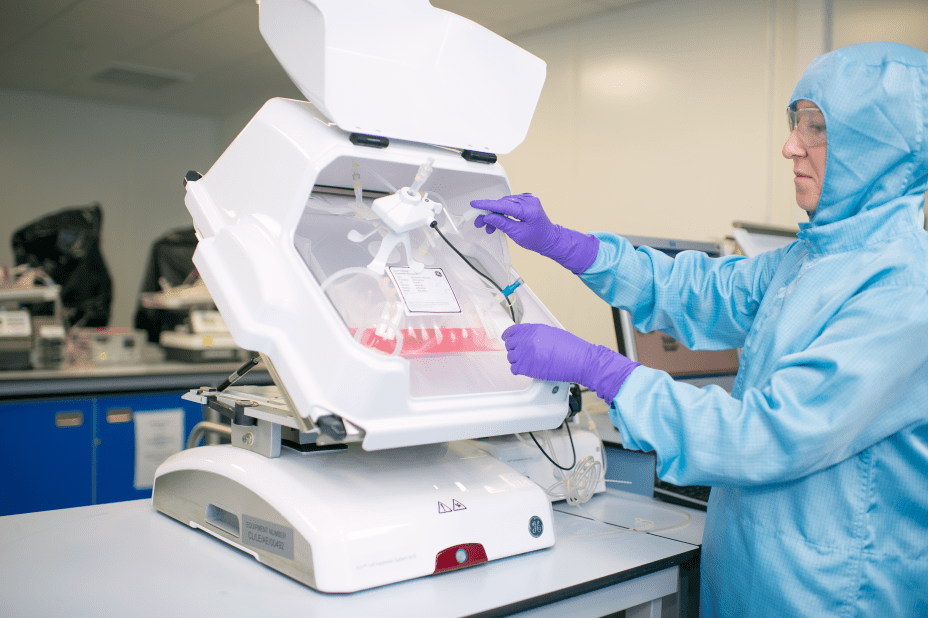
- Creating a fully automated robotic system will bring industrial scale reliability to cell therapy manufacturing while providing speed and flexibility
- Automation readily used in other industries is now being tested in the cell therapy workflow
- Technology has the potential to transform global healthcare as more cell therapies receive regulatory approval around the world
With cell therapies being approved by regulatory bodies in the United States, the European Union, Canada, Japan and Australia1, and hundreds2 more in clinical development globally, there is an immediate need to industrialize and automate the workflow. Combining Cytiva’s industry knowledge and technology with Multiply Labs’ automation and robotics expertise, the companies will develop a proof-of-concept robotic system that will accelerate the manufacture of cell therapies on an industrial scale.
Catarina Flyborg, Vice President, Cytiva, says: “Cell therapies have the potential to transform global healthcare, but their true potential will not be realized until the manufacturing workflow is fully automated. Our collaboration with Multiply Labs will enable us to develop a system that will advance and accelerate the commercial manufacturing of these novel therapeutics to help get them to the patients who need them most.”
Multiply Labs’ technical approach is based on a first-of-its-kind robotic cluster, a cloud-controlled manufacturing system that consists of multiple modules organized around a shared transfer system. This approach enables greater output, increased safety, more reproducibility, greater flexibility and easier GMP qualification for drug developers. Multiply Labs has successfully deployed a fully automated robotic cluster for the manufacture of personalized therapies made with combinations of small molecules.
Fred Parietti, co-founder and CEO, Multiply Labs, says: “Advanced robotics has been successfully applied to numerous high-value, high-scale manufacturing processes, from automotives to semiconductors. Combining Cytiva’s process expertise and market-leading technology with our robotics hardware and software expertise has the potential to radically accelerate the development and adoption of these life-saving drugs.”
There were 440 ongoing cell-based and cell therapy clinical trials worldwide in 2020 creating an immediate need to develop an industrialized manufacturing system that can support potential regulatory approvals and the commercialization of these novel therapeutics.
Most Read
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











