Neurosteer Announces FDA Clearance of the Neurosteer EEG Brain Monitoring Platform
04 November 2022 | Friday | News
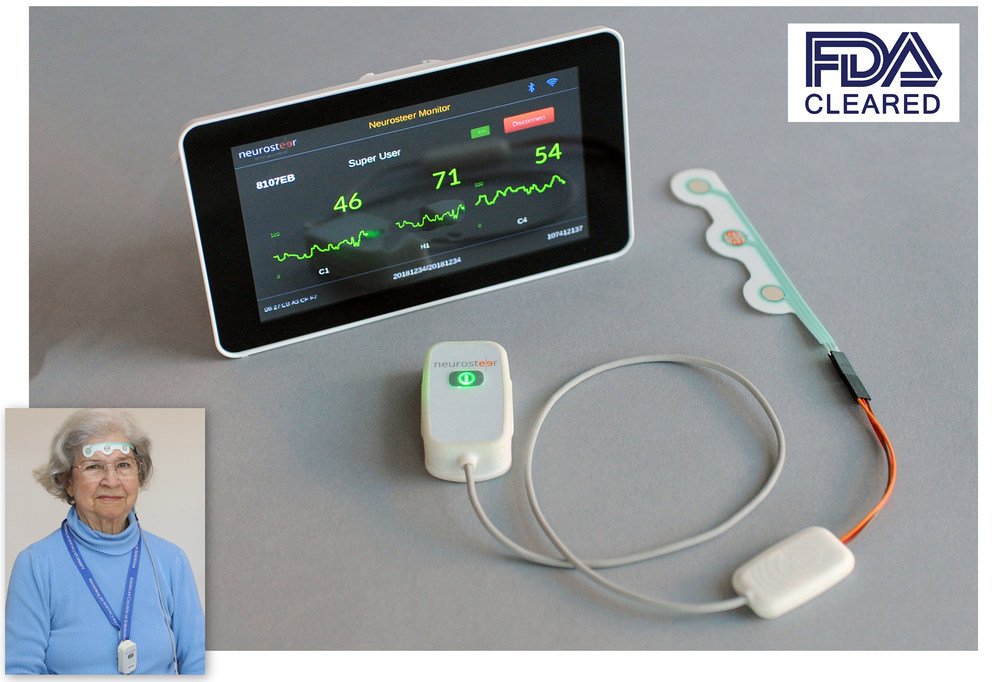
Image Source : Public Domain
Neurosteer Inc. announced the FDA clearance of its Neurosteer® single-channel EEG brain monitoring platform. This clearance allows Neurosteer's unobtrusive multi-purpose system to be used in a broad range of clinical settings. In the ICU, it can offer continuous brain monitoring to support critical interventions. In a doctor's office, it can aid in the early detection of pre-symptomatic cognitive decline, including Alzheimer's, Parkinson's and dementia. And in pharma drug trials, it can assist in the rapid and cost-effective mass screening of subjects who may be suffering from neurodegenerative disorders.
Neurosteer advances the century-old EEG into the 21st century, using an adhesive electrode strip connected to a pocket-sized sensor that wirelessly transmits EEG data to the cloud for advanced signal processing. Using just a single adhesive forehead strip, Neurosteer's innovative solution offers the traditional EEG frequency bands while also being cleared by the FDA to include several novel "brain metrics" visual representations.
These brain metrics rely on Neurosteer's advanced signal processing technology and may assist trained medical staff in making neurological diagnoses. In addition, affixing the adhesive strip to the forehead avoids interference from the hair, and using dry gel supports unattended operation for up to 12 hours. Finally, the system includes a noninvasive assessment with auditory prompts that can aid in the early detection of brain deterioration.
Dr. Amitai Bickel, Surgeon at Western Galilee Hospital, commented, "After working with the Neurosteer EEG system for several years, I've realized what an advantage it is to have a compact, easy-to-apply EEG brain monitor that can be used for real-time continuous monitoring. Neurosteer's innovative platform has the potential to revolutionize the standard of care in managing brain health."
Dr. Nathan Intrator, Founder and CEO of Neurosteer, said, "We are extremely pleased that the FDA has cleared our portable and affordable EEG system, and moreover, recognized the potential clinical value of our brain metrics visual representations. This is an important step in achieving our goal of making brain monitoring and assessment widely available to all populations."
Most Read
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Avantor’s New CEO Ligner Aims to Unlock Global Potential and Deliver Shareholder Value
- AstraZeneca Commits $50 Billion to U.S. Expansion by 2030 in Biggest-Ever Global Investment
- Thermo Fisher, SAMRC, and South Africa’s Department of Science and Innovation Launch CATIR to Nurture Next-Gen Scientists
- Cube Biotech Appoints Former Sartorius CEO Dr. Joachim Kreuzburg to Board of Directors
- FDA’s AI Transition Marks a Turning Point in Drug Review: Industry Faces Pressure to Adapt Amid 20% Workforce Cut
- WuXi XDC Completes Mechanical Build of Singapore Bioconjugate Manufacturing Hub
News
Editor Picks











