Singapore Grants Approval for INDICAID COVID-19 Rapid Antigen Test
27 January 2022 | Thursday | News
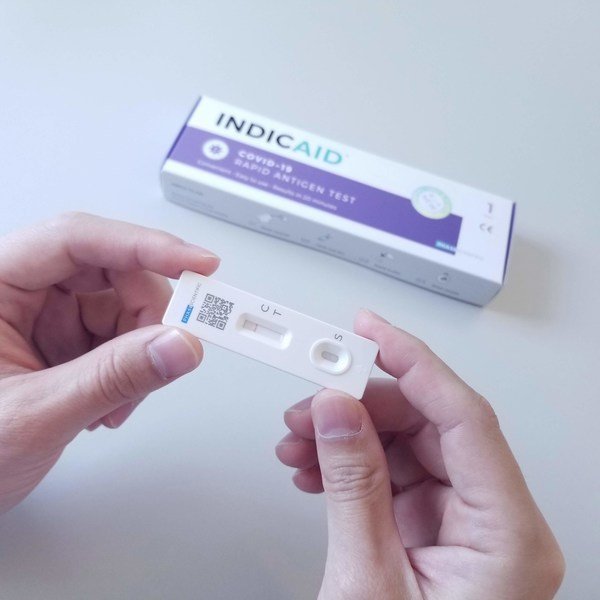
INDICAID COVID-19 Rapid Antigen Test - Accurate and easy-to-use
PHASE Scientific International LTD (PHASE Scientific), today announced that its INDICAID™ COVID-19 Rapid Antigen Test (INDICAID™) has received authorisation from the Health Sciences Authority of Singapore, which can make it available for use by individual citizens in the city as a self-testing tool for rapid detection of SARS-CoV-2 antigens.
Dr Ricky Chiu Yin-to, Founder and CEO of PHASE Scientific, welcomes the authorisation of INDICAID™ for use in Singapore. "The COVID-pandemic has entered into another critical phase with the emergence of the Omicron variant and a new wave of infections. The faster one can know if he or she is COVID-19 infected, the sooner he or she can isolate immediately and get appropriate care."
"The Singapore Government has been encouraging regular self-testing for all, including fully vaccinated individuals. I strongly support this arrangement as it is a matter of public responsibility. Antigen rapid test is convenient to use and can provide fast results. It is the best option for large-scale screening and is an effective way to help limit the spread of SARS-CoV-2," says Dr Chiu.
INDICAID™ is a lateral flow immunoassay designed for the qualitative detection of SARS-CoV-2 antigens in direct nasal swab samples. It has been validated in the world's largest clinical trial for a product of its kind with over 22,000 samples from the community in Hong Kong that were asymptomatic. The results demonstrated that INDICAID™ had excellent sensitivity and specificity and was a high-performing product for fast and effective SARS-CoV-2 screening.
INDICAID™ is developed in Hong Kong and is the first antigen rapid test product in the Greater China region to have obtained the US Food and Drug Administration Emergency Use Authorization. It is easy-to-use with no special equipment or facilities needed and the result is available within as fast as 20 minutes. The product is used globally for regular SARS-CoV-2 screening to meet various emergency testing requirements. To date, sales have been made to 30 countries with over 20 million kits sold.
Most Read
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











