BioPharma Drug Approval
Senhwa’s CX-4945 Shows Promising Disease Control and Durability in Refractory Basal Cell Carcinoma
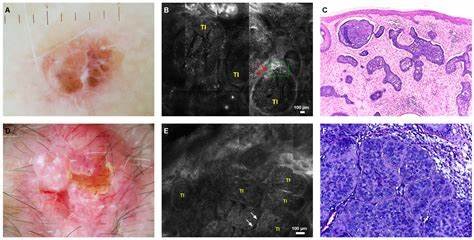
Basal Cell Carcinoma CSR Highlights CX-4945 Monotherapy: Progression-free survival (PFS) exceeded 21 months in two patients. Optimal Treatment Res...
April 03, 2025 | News
Yingli Pharma Receives FDA Clearance to Launch Global Phase 3 Trial of Linperlisib for Relapsed/Refractory PTCL
Shanghai Yingli Pharmaceutical Co., Ltd. (Yingli Pharma), a clinical stage biotechnology company developing oral small molecule drugs for cancer, metabolic...
April 02, 2025 | News
Ascletis’ ASC30 SQ Injection Shows 36-Day Half-Life in Phase Ib Obesity Trial
-Ultra-long-acting subcutaneous (SQ) injection formulation of small molecule ASC30 demonstrated a 36-day half-life in patients with obesity, supp...
April 01, 2025 | News
Luye Pharma’s Twice-Weekly Rivastigmine Patch Approved in Japan for Alzheimer’s
Luye Pharma Group announced that marketing approval for its innovative formulation Rivastigmine Twice Weekly Transdermal Patch has been granted by the Japa...
April 01, 2025 | News
IASO Bio Secures First Approval Outside Mainland China for Equecabtagene Autoleucel in Macau
IASO Biotherapeutics ("IASO Bio"), a biopharmaceutical company focused on the discovery, development, manufacturing, and commercialization of innovative ce...
March 31, 2025 | News
Langhua Pharmaceutical Receives FDA EIR Following Successful cGMP Inspection for the Fourth Time
Zhejiang Langhua Pharmaceutical Co., Ltd. ("Langhua Pharmaceutical"), a wholly-owned subsidiary of Viva Biotech Holdings ("Viva Biotech"), underwent a cGMP...
March 31, 2025 | News
ZEISS and DORC Secure NMPA Approval in China for ILM-Blue®, Advancing Retinal Surgery Standards
ZEISS Medical Technology announced today that the ILM staining dye ILM-Blue® from DORC (Dutch Ophthalmic Research Center (International) B.V...
March 28, 2025 | News
CJ BIO’s BiomeNrich™ POST M005 Becomes First Akkermansia muciniphila-Based Ingredient to Gain FDA NDIN Acknowledgment
CJ BIO's BiomeNrich™ POST M005 is the first Akkermansia muciniphila-based ingredient to receive New Dietary Ingredient Notification (NDIN) ...
March 28, 2025 | News
GSK’s Omjjara Receives Local Approval in Singapore for Myelofibrosis Patients with Moderate to Severe Anaemia
Approval is for use in myelofibrosis patients with moderate to severe anaemia who are JAK-naive or previously treated with ruxolitinib Nearly all myelof...
March 25, 2025 | News
SineuGene Therapeutics Receives FDA IND Clearance for First-in-Class ALS Gene Therapy SNUG01
SineuGene Therapeutics Co., Ltd. ("SineuGene"), a clinical-stage biotech company pioneering innovative therapies for neurological disorders, announce...
March 25, 2025 | News
Sungen Biomedical Secures FDA Fast Track Designation for AMI Drug Candidate SGC001
Sungen Biomedical—an innovative biopharmaceutical company incubated by Beijing Hotgen Biotech Co., Ltd. (SH.688068)—received Fast Track Designa...
March 25, 2025 | News
TiumBio and Hansoh Pharma Gain Clinical Trial Approval in China for Merigolix in ART-Related Controlled Ovarian Stimulation
Clinical Trial Approval of merigolix (TU2670, HS-10518) for the inhibition of premature LH (luteinizing hormone) surges in women undergoing COS (con...
March 21, 2025 | News
LISCure Biosciences Secures World’s First Regulatory Approval for Hair Health Probiotic, Mobiome®, in South Korea
LISCure Biosciences Inc. ("LISCure") announced on the 2025 Feb that it has received approval from the Korean Ministry of Food and Drug Safety (KM...
March 21, 2025 | News
Shanghai Henlius Secures FDA Orphan Drug Designation for HLX22 in Gastric Cancer
Shanghai Henlius Biotech, Inc. (2696.HK) announced that the U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) for...
March 20, 2025 | News
Most Read
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News