BioPharma Drug Approval
China’s D3 Bio Secures FDA Breakthrough and Orphan Drug Designations for KRAS G12C Inhibitor D3S-001
Breakthrough Therapy Designation was granted for the treatment of adult patients with KRAS G12C-mutated locally advanced or metastatic non-small cel...
August 29, 2025 | News
MS Pharma Inaugurates Middle East’s First SFDA-Approved Biologics Manufacturing Facility In Saudi Arabia
MS Pharma inaugurates Middle East's first biologics manufacturing facility in Saudi Arabia, SFDA-approved and built to meet EMA and F...
August 28, 2025 | News
Abbott Secures Thailand Approval For Denosumab Biosimilar Expanding Access To Osteoporosis Treatment
Abbott denosumab biosimilar has received regulatory approval in Thailand, expanding access to advanced therapies for osteoporosis and cancer-related bone l...
August 27, 2025 | News
BioDlink Secures Indonesian Approval for Bevacizumab, Accelerating ASEAN and Global Expansion
BioDlink announced that its Bevacizumab Injection has obtained marketing authorization from Indonesia's National Agency of Drug and Food Con...
August 22, 2025 | News
Everest Medicines’ Etrasimod (VELSIPITY®) Receives Strong Recommendation in Updated ACG Clinical Guideline for Ulcerative Colitis
Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the discovery, clinical development, manufacturing, a...
August 18, 2025 | News
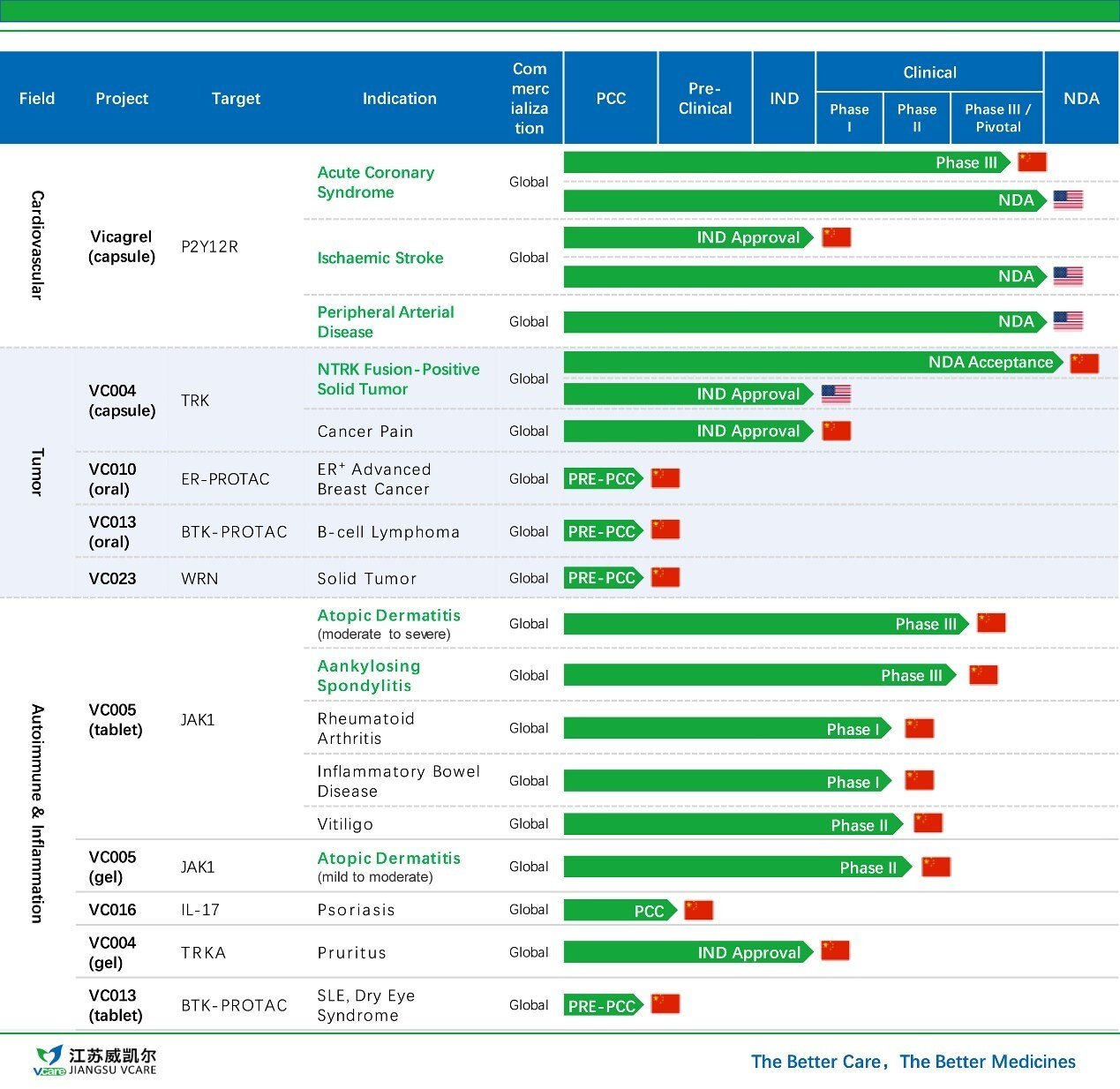
Jiangsu Vcare and Huadong Medicine Form Exclusive Partnership for Commercialization of VC005 Tablets in China
Jiangsu Vcare Pharmatech Co., Ltd. (Jiangsu Vcare) announced that it has entered into an exclusive strategic partnership with Huadong Medicine (H...
August 18, 2025 | News
Shilpa Medicare Becomes World’s First to Gain Approval for NorUDCA, Pioneering Treatment for Over a Billion NAFLD Patients
NAFLD affects 1 in 4 people globally (1.2 billion), with 188 million patients in India—most undiagnosed until irreversible damage...
August 14, 2025 | News
Everest Medicines Secures Full Taiwan Approval for NEFECON® in IgA Nephropathy
Everest Medicines (HKEX 1952.HK) recently announced the Taiwan Food and Drug Administration (TFDA) has approved the supplementary application for NEFECON(R...
August 12, 2025 | News
Bracco Imaging’s SonoVue® Gains NMPA Approval for HyCoSy Use in Infertility Diagnosis in China
Bracco Imaging, a global leader in diagnostic imaging,announced that its ultrasound contrast agent SonoVue® has been approved by the China's&...
August 06, 2025 | News
Chipscreen Biosciences Receives FDA IND Approval for Brain-Penetrant Aurora B Inhibitor CS231295
Shenzhen Chipscreen Biosciences Co., Ltd. ("Chipscreen Biosciences") announced that its wholly owned subsidiary, Chipscreen Biosciences (USA) Ltd., has rec...
August 04, 2025 | News
Mabwell’s CDH17-Targeting ADC 7MW4911 Receives IND Clearance in China and U.S.
Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced that its proprietary CDH17-targeting antibody-drug ...
August 04, 2025 | News
BON Natural Life Launches AI-Powered Drug Discovery Platform, Advancing TCM-Based Pharma Innovation
Bon Natural Life Limited (Nasdaq: BON) ("BON" or "the Company"), a leading provider of bio-ingredient solutions for the natural health and personal care in...
July 31, 2025 | News
Sirtex Secures FDA Approval for SIR-Spheres® Y-90 in Treating Unresectable Hepatocellular Carcinoma
Sirtex Medical ("Sirtex"), a leading manufacturer of interventional oncology solutions, today announced that the U.S. Food and Drug Administration (F...
July 08, 2025 | News
Celltrion Launches STOBOCLO® and OSENVELT® Biosimilars in the U.S. for Comprehensive Bone Health and Oncology Care
STOBOCLO® (denosumab-bmwo) and OSENVELT® (denosumab-bmwo) are approved by FDA for all indications of PROLIA® (denosumab) and X...
July 08, 2025 | News
Most Read
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News