BioPharma Drug Approval
ImmunityBio Secures First International Approval for ANKTIVA® in the UK for Bladder Cancer Treatment
ANKTIVA, a first-in-class, lymphocyte-stimulating agent, works synergistically with BCG to activate and proliferate natural killer (NK) and T cells, he...
July 08, 2025 | News
Clasado Biosciences Gains TGA Approval for Bimuno® GOS in Australia’s Therapeutic Supplement Market
Clasado Biosciences has announced that its prebiotic ingredient Bimuno® GOS is now available for use in Therapeutic Goods Administration (TGA)-listed p...
July 07, 2025 | News
Dizal Secures FDA Approval for ZEGFROVY, the First Targeted Oral Therapy for EGFR Exon20ins NSCLC
ZEGFROVY is the only approved targeted oral treatment for NSCLC with EGFR exon20ins Approval follows the U....
July 03, 2025 | News
Switzerland’s RDP Pharma and Singapore’s A*STAR EDDC Join Forces to Develop Precision Autoimmune Therapies
RDP Pharma AG, a Swiss biotechnology company focused on next-generation protein degradation therapeutics, and the Experimental Drug Development...
July 02, 2025 | News
Innovent Biologics Secures NMPA Approval for World’s First Dual GCG/GLP-1 Receptor Agonist Mazdutide for Weight Management in China
Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality me...
June 30, 2025 | News
Daiichi Sankyo and AstraZeneca’s DATROWAY Receives U.S. FDA Accelerated Approval for EGFR-Mutated Lung Cancer
Based on TROPION-Lung05 phase 2 trial results and supported by data from the TROPION-Lung01 phase 3 trial Second U.S. approval for Daiichi Sankyo and ...
June 24, 2025 | News
RemeGen’s Telitacicept Secures EMA Orphan Drug Designation for Myasthenia Gravis, Advancing Global Development
RemeGen Co., Ltd. ("RemeGen", stock symbols: 688331.SH/09995.HK) announced that telitacicept (RC18; brand name: 泰爱®) has received Orphan Drug Desig...
June 18, 2025 | News
Harrow Secures Exclusive U.S. Rights to BYQLOVI for Post-Surgical Eye Care Following FDA Approval
Harrow (Nasdaq: HROW), a leading North American eyecare pharmaceutical company, and Taiwan-based Formosa Pharmaceuticals ("Formosa", 6838.TW), today a...
June 10, 2025 | News
ZEISS CLARUS® 700 Receives NMPA Approval in China, Enhancing Retinal Diagnostics
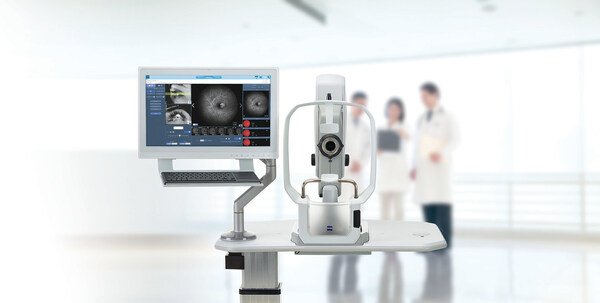
ZEISS Medical Technology announced that the CLARUS® 700 from ZEISS has received National Medical Products Administration (NMPA) approval in China,...
June 06, 2025 | News
Innovent and HUTCHMED’s Sintilimab-Fruquintinib Combo Accepted for NMPA Review in Advanced Renal Cell Carcinoma
Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality me...
June 05, 2025 | News
Innovent Secures Second NMPA Breakthrough Therapy Designation for IBI363 in Advanced Lung Cancer
Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures, and commercializes high-quality m...
June 05, 2025 | News
JW Therapeutics Secures Macao Approval for CAR-T Therapy Carteyva®, Marking First Overseas Market Entry
JW Therapeutics (HKEx: 2126), an independent and innovative biotechnology company focusing on developing, manufacturing and commercializing cell immunother...
June 05, 2025 | News
Akeso’s Cadonilimab Secures NMPA Approval for First-Line Treatment of Cervical Cancer
Akeso, Inc. is pleased to announce that the National Medical Products Administration (NMPA) has approved the company's first- in-class PD-1/CTLA-4 bispecif...
June 05, 2025 | News
Singapore Approves Astellas’ VEOZA™ (Fezolinetant) for Moderate to Severe Menopausal Vasomotor Symptoms
Astellas Singapore has announced that the Health Sciences Authority (HSA) of Singapore has approved VEOZA™ (fezolinetant) 45 mg for the treatment of ...
June 04, 2025 | News
Most Read
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News