HistoSonics Announces Agreement with GE Healthcare
24 May 2022 | Tuesday | News
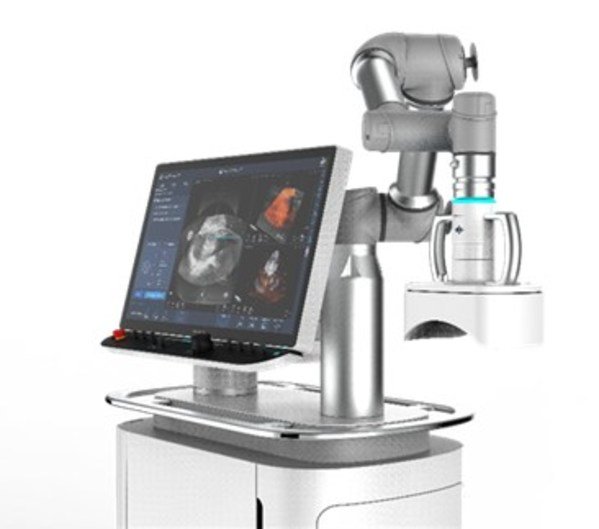
HistoSonics Inc., developer of a completely non-invasive platform using the science of histotripsy, today announced an agreement formalizing ongoing efforts to use GE Healthcare's LOGIQ E10 Series ultrasound imaging platform to power the real time visualization features of HistoSonics' novel sonic beam therapy. As part of the agreement and upon market authorization, HistoSonics will distribute GE Healthcare's LOGIQ E10 Series on a one-to-one basis with its breakthrough liver therapy system.
HistoSonics' Edison™ system, currently in development, uses the novel science of histotripsy to non-invasively destroy targeted liver tissue. HistoSonics intends to utilize GE Healthcare's LOGIQ E10 Series, currently the most technologically advanced ultrasound platform for guiding radiology interventions, to provide treating physicians with continuous visualization for key and unique elements of the histotripsy therapy procedure, including planning, monitoring, and immediate post-treatment verification. This agreement is aimed to support HistoSonics' efforts to launch their EdisonTM system leveraging their deep domain expertise along with GE Healthcare's world-class ultrasound imaging technologies, digital infrastructure, data analytics and clinical decision support capabilities.
"We are very excited to formalize our imaging partnership with GE Healthcare, which is a key part of bringing our transformative therapy platform, and an entirely new treatment option, to the clinic and to patients," said Josh Stopek, HistoSonics Vice President of R&D. "We've developed a very collaborative relationship with GE Healthcare and look forward to expanding our efforts to realize the full potential of histotripsy across clinical applications, specialties, and care settings."
HistoSonics' non-invasive platform combines advanced imaging and proprietary software to deliver patient specific treatments using histotripsy to mechanically destroy and liquify targeted tissues at a sub-cellular level. The company believes the novel mechanism of action of their proprietary technology may offer significant advantages to patients, including precise and predictable treatment zones with equivalent treatment effect throughout the entire treated volume. Early clinical and pre-clinical results also suggest that histotripsy largely preserves critical structures such as the liver capsule, and larger vessels and bile ducts within or adjacent to the treated volume of tissue. Additionally, histotripsy enables the treating physicians to monitor the destruction of tissue under continuous real-time visualization and control, unlike any modality that exists today.
The agreement between GE Healthcare and HistoSonics comes as HistoSonics continues enrollment in their U.S. and European #HOPE4LIVER Trials, evaluating the safety and efficacy of histotripsy for the destruction of targeted primary or metastatic liver tumors. Additionally, the company recently was awarded "Breakthrough Device Designation" by the FDA for histotripsy of liver tissue, validating the company's vision that histotripsy has the potential to provide advantages over existing therapies such as surgery, radiation therapy and thermal ablation.
The HistoSonics System is investigational and is not available for sale in the United States or Europe. It is limited to investigational use in the approved IDE and European studies.
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











