Shanghai Ark Biopharmaceutical Secures NMPA Approval For Azstarys ADHD Treatment In China
07 January 2026 | Wednesday | News
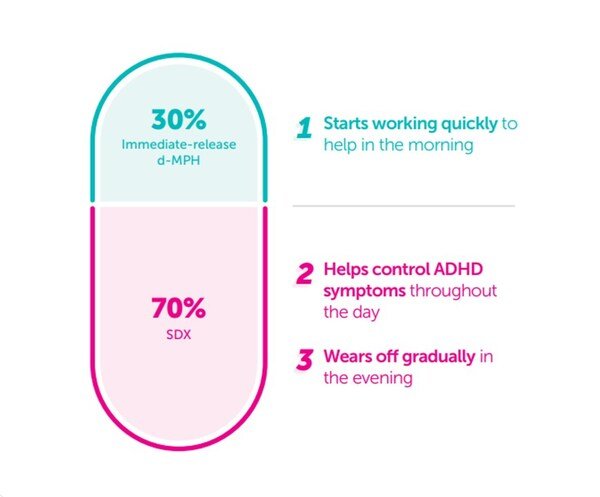
Figure 1. Each Azstarys® capsule contains 70% SDX and 30% d-MPH
Shanghai Ark Biopharmaceutical Co., Ltd. ("ArkBio") announced that China's National Medical Products Administration (NMPA) has approved the New Drug Application for Serdexmethylphenidate Chloride and Dexmethylphenidate Hydrochloride Capsules (Azstarys®) for treatment of Attention Deficit Hyperactivity Disorder (ADHD) in patients 6 years of age and older in China.
Azstarys® is a central nervous system stimulant indicated for the treatment of ADHD. Each once-daily capsule contains serdexmethylphenidate (SDX), a prodrug of dexmethylphenidate, co-formulated with immediate-release dexmethylphenidate (d-MPH) in a fixed ratio designed to provide both rapid and extended symptom control.After ingestion, the immediate-release d-MPH component is rapidly absorbed to provide early onset of effect, while SDX is gradually converted to d-MPH in the lower gastrointestinal tract, supporting sustained therapeutic exposure throughout the day. Azstarys® was approved by the U.S. FDA in March 2021 as a once-daily treatment option for patients with ADHD 6 years of age and older.
ADHD is a common, chronic neurodevelopmental disorder that typically begins in childhood and may persist into adulthood. It is primarily characterized by age-inappropriate levels of inattention and/or hyperactivity-impulsivity. In China, the prevalence of ADHD in children and adolescents is estimated at approximately 6.4%, affecting over 23 million individuals. Studies have shown that symptoms persist into adolescence for 60%-80% of patients, and into adulthood for roughly half of cases. Current ADHD treatment options in China are limited, and currently available medications do not fully meet clinical needs in terms of both efficacy and safety for many patients. Some patients discontinue treatment due to suboptimal response, side effects, or dosing inconvenience, highlighting the need for additional therapeutic options.
Robust Clinical Data Addresses Unmet Need: In a pivotal Phase III clinical trial in Chinese patients with ADHD, Azstarys® met both the primary and key secondary endpoints. The study demonstrated statistically significant and clinically meaningful improvements in core ADHD symptoms compared to placebo at all assessment time points. With limited treatment options in China and historical supply constraints for traditional single-agent therapies, the approval of this innovative combination product offers an important new choice for clinicians and patients.
Unique Dual Mechanism for Rapid Onset and All-Day Coverage: Azstarys® combines immediate-release and prodrug-based extended-release technologies. Its 'rapid onset + full-day coverage' profile positions it to reshape the ADHD treatment paradigm in China. As the first ADHD medication in China to deliver both rapid onset and sustained symptom control, it fills a key clinical gap and has the potential to become a preferred treatment option.
Professor Yi Zheng, Chief Expert at Beijing Anding Hospital, Capital Medical University, and Lead Principal Investigator of the Azstarys® Phase III trial in China, commented: "ADHD is one of the most common neurodevelopmental disorders in childhood, with potential impact lasting into adulthood, posing long-term challenges to learning, social functioning, and family life. While the prevalence in Chinese children is around 6.4%, treatment options—especially innovative ones balancing efficacy and safety—remain insufficient. The approval of Azstarys® provides clinicians with a new therapeutic tool. We anticipate its real-world application will help improve the treatment landscape for ADHD patients in China, offering new hope particularly for those with suboptimal response or tolerance to existing therapies."
Professor Jing Liu, Director of the Child Psychiatry Center, Peking University Sixth Hospital, and Lead Principal Investigator of the Azstarys® Phase III trial in China, added: "We are committed to advancing standardized diagnosis and treatment for pediatric mental disorders in China. Comprehensive intervention is essential for ADHD, with pharmacotherapy being a foundational component. The approval of a new drug not only increases options but also prompts deeper reflection on optimizing treatment strategies. We hope the introduction of Azstarys® will enrich ADHD treatment approaches and contribute to better long-term outcomes, which is the ultimate goal of our clinical research."
Azstarys® is a registered trademark of Corium, LLC in the United States.
Most Read
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Avantor’s New CEO Ligner Aims to Unlock Global Potential and Deliver Shareholder Value
- AstraZeneca Commits $50 Billion to U.S. Expansion by 2030 in Biggest-Ever Global Investment
- Thermo Fisher, SAMRC, and South Africa’s Department of Science and Innovation Launch CATIR to Nurture Next-Gen Scientists
- Cube Biotech Appoints Former Sartorius CEO Dr. Joachim Kreuzburg to Board of Directors
- FDA’s AI Transition Marks a Turning Point in Drug Review: Industry Faces Pressure to Adapt Amid 20% Workforce Cut
- WuXi XDC Completes Mechanical Build of Singapore Bioconjugate Manufacturing Hub
News
Editor Picks











