Biologics
Antengene And Junshi Biosciences Enter Clinical Collaboration To Advance ATG 037 And JS207 Combination In Solid Tumors
Antengene Corporation Limited ("Antengene", SEHK: 6996.HK) , a leading innovative, commercial-stage global biotech company dedicated to discovering, ...
February 26, 2026 | News
SteinCares And Shilpa Biologicals Sign Strategic Licensing Agreement To Commercialise Biosimilar Across Latin America
New partnership combines Shilpa Biologicals' development and manufacturing capabilities with SteinCares' regional commercialization platform Collaborati...
February 26, 2026 | News
Etherna And Almirall Advance LAD116 mRNA LNP Therapy Into IND Enabling Studies For Non Melanoma Skin Cancer
etherna, a leading provider of cutting-edge mRNA and lipid nanoparticle (LNP) technology across the biotech and pharma industry, announces that one o...
February 25, 2026 | News
iRegene Advances Global Cell Therapy Programme With Key Parkinson’s And MSA Clinical Milestones
The global race to treat neurodegenerative diseases has reached a new tempo as iRegene Therapeutics pushes its cell therapy pipeline into key clinical...
February 25, 2026 | News
Bioxytran Initiates University Of Georgia Collaboration Under 100 Million Dollar Grant To Target H5N1 Bird Flu
Bioxytran, Inc. (OTCQB: BIXT), a clinical-stage biotechnology company developing breakthrough antiviral treatments, announced it has initiated a research c...
February 24, 2026 | News
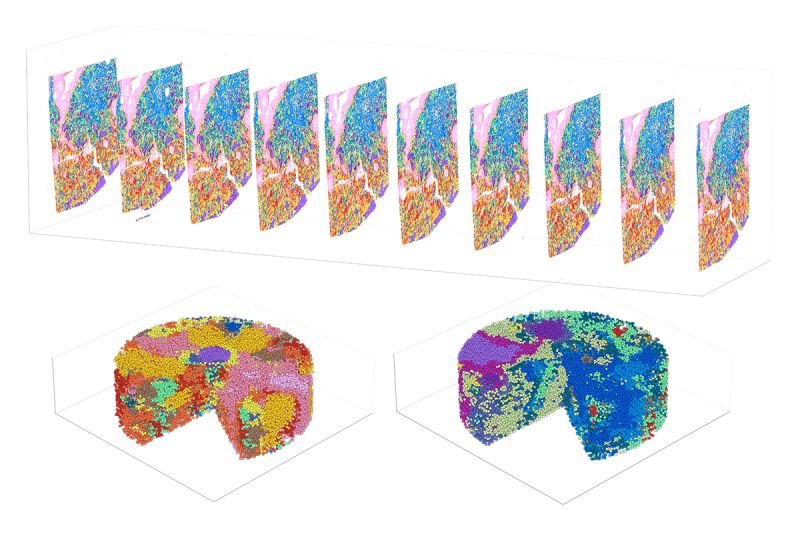
Singular Genomics Commercially Launches G4X to Scale Spatial Multiomics for Precision Medicine
Singular Genomics, a company delivering high-throughput spatial technologies to advance precision medicine, announced the commercial launch...
February 24, 2026 | News
AVEO Oncology Selects Ficlatuzumab Dose for Phase 3 FIERCE-HN Trial in Head and Neck Cancer
– Global enrollment continues in the Phase 3 registrational studyfollowing the planned first interim analysis for patients with HPV-negati...
February 24, 2026 | News
Hansa Biopharma Announces FDA Acceptance of Imlifidase Biologics License Application
Hansa Biopharma AB, ("Hansa" or "the Company"), announced that its Biologics License Application (BLA) for imlifidase has been accepted by the U.S. F...
February 24, 2026 | News
Lunai Bioworks Secures Foundational U.S. Patent to Advance AI-Driven Precision Medicine
Foundational Patent Strengthens AI Moat Across Multimodal Data Standardization, Disease Stratification and Gene Mapping Lunai Bioworks, Inc. (NASDAQ: ...
February 24, 2026 | News
Catalent And S Biomedics Partner To Advance Stem Cell Therapy For Parkinsons Disease
Catalent and S.Biomedics announced a strategic partnership to support the development and manufacturing of TED‑A9, S.Biomedics’ allogeneic pluripot...
February 23, 2026 | News
Asahi Kasei Pharma Advances AK1960 Into Phase I For Refractory Kidney Diseases
Asahi Kasei Pharma, a global provider of healthcare and pharmaceutical solutions, in collaboration with Alchemedicine, has announced that its novel Endothe...
February 23, 2026 | News
ANKTIVA Plus BCG Secures European Commission Authorization As First Immunotherapy For BCG Unresponsive NMIBC
ANKTIVA plus BCG, with a 71% complete response rate, is the first immunotherapy to receive marketing authorization in Europe for non-muscle invasive blad...
February 20, 2026 | News
Mammotome Introduces In Room MRI Guided Breast Biopsy System To Improve Clinical Workflow
Mammotome, a Danaher company, announces the Mammotome Prima™ MR Dual Vacuum-Assisted Breast Biopsy System, the industry's first system designed t...
February 19, 2026 | News
Pharmacelera Secures €6 Million To Expand US Footprint And Advance Quantum AI Drug Discovery Platform
Round led by Heran Partners and joined by Clave Capital, Inveready and Bio&Tech Smart Capital Financing will enable Pharmacelera to build ongoi...
February 19, 2026 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News