FDA Clears DyAnsys Neurostimulation Device First Relief to treat Diabetic Neuropathic Pain
18 July 2022 | Monday | News
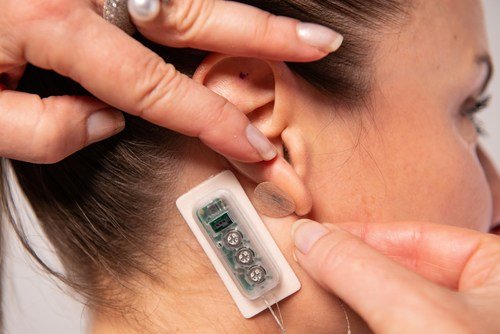
The First Relief percutaneous neurostimulation device can be used to treat pain related to diabetic neuropathy. It is applied behind the ear and delivers continuous pulses of a low-level electrical current over several days.
The wearable device placed on the ear administers continuous pulses of a low-level electrical current over several days.
With the FDA clearance of First Relief, this device can be used to treat pain related to diabetic neuropathy.
"We are excited to have the FDA clearance of First Relief so that this device, which has been proven effective, can now be used to treat patients who have been experiencing pain related to diabetic neuropathy, said DyAnsys CEO Srini Nageshwar. "First Relief offers a significant treatment option without drugs or narcotics."
The approval was based on a study that tested First Relief against a placebo and another device previously cleared by the US Food and Drug Administration.
The study was conducted at the Jeevak Multispeciality Hospital in Warangal, India, renowned for the treatment of diabetes. The single center, three-arm, randomized, controlled, parallel assignment, double blinded, prospective study involved 63 patients age 30 to 74.
The devices were applied on a bi-weekly basis for 16 weeks. The primary efficacy endpoint was pain intensity measured through Visual Analog Scale (VAS) score and the secondary efficacy endpoints are vibration perception threshold (VPT) value, insomnia severity index (ISI), overall neuropathy limitations scale (ONLS), Hamilton rating scale for anxiety.
The VAS pain score analysis showed a significant reduction in the pain score of patients being treated with First Relief from start of the treatment to the end. This improvement persisted throughout the 90-day follow-up, suggesting that the treatment was a long-term improvement in neuropathic pain and not a short-term improvement. The secondary outcome measures (VPT, Insomnia, ONLS and HAM) also showed similar improvements to the pain score, showing significant improvement in sleep and mood as the neuropathic pain decreased.
No complications or adverse events were observed in any of the subjects during the study period.
DyAnsys Inc. is a global company headquartered in California with subsidiaries in Switzerland and India. DyAnsys provides advanced medical diagnostic and monitoring systems to clinicians in individual practices and hospitals. DyAnsys, ANSiscope. First Relief, Primary Relief and Drug Relief are registered trademarks, of DyAnsys, Inc.
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











